Molds are multinucleated, filamentous fungi composed of hyphae. A hypha is a branching, tubular structure from 2-10 µm in diameter and is usually divided into cell-like units by crosswalls called septa. The total mass of hyphae is termed a mycelium. The portion of the mycelium that anchors the mold and absorbs nutrients is called the vegetative mycelium ; the portion that produces asexual reproductive spores is termed the aerial mycelium. (See Fig. 1.)
Molds possess a rigid polysaccharide cell wall composed mostly of chitin and, like all fungi, are eukaryotic (See Fig. 2.) Molds reproduce primarily by means of asexual reproductive spores such as conidiospores, sporangiospores, and arthrospores. These spores are disseminated by air, water, animals or objects and upon landing on a suitable environment, germinate and produce new hyphae. (See Fig. 1). Molds may also reproduce by means of sexual spores such as ascospores and zygospores, but this is not common. The form and manner in which the spores are produced, along with the appearance of the hyphae and mycelium, provide the main criteria for identifying and classifying molds.
Fig. 1: Asexual Reproduction in Molds
Fig. 2: Segment of a Mold Hypha Showing Eukaryotic Nature
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
A. COMMON MOLDS
To illustrate how morphological characteristics such as the type and form of asexual reproductive spores and the appearance of the mycelium may be used in identification, we will look at three common non-pathogenic molds.
The two most common types of asexual reproductive spores produced by molds are conidiospores and sporangiospores. Conidiospores are borne externally in chains on an aerial hypha called a conidiophore. (See Fig. 3.); sporangiospores are produced within a sac or sporangium on an aerial hypha called a sporangiophore. (See Fig. 4.)
Fig. 3: Fungal Conidiospores
Fig. 4: Fungal Sporangiospores within a Sporangium
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Penicillium and Aspergillus are examples of molds that produce conidiospores. Penicillium is one of the most common household molds and is a frequent food contaminant. The conidiospores of Penicillium (see Fig. 5A and Fig. 5B) usually appear grey, green, or blue (See Fig. 5C) and are produced in chains on finger-like projections called phialides coming off of the conidiophore. Fig. 5D shows conidiospores of Penicillium as seen with a scanning electron microscope.
Fig. 5A: Conidiospores of Penicillium
Fig. 5B: Conidiospores of Penicillium
Fig. 5C: Penicillium Growing on Saboraud Dextrose Agar
Fig. 5D: Scanning Electron Micrograph of Penicillium showing Conidiospores
> 
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
By AJC1 [CC BY-SA 4.0-3.0-2.5-2.0-1.0 (https://creativecommons.org/licenses/by-sa/4.0-3.0-2.5-2.0-1.0)], via Wikimedia Commons
Aspergillus is another common contaminant. Although usually non-pathogenic, it may become opportunistic in the respiratory tract of a compromised host and, in certain foods, can produce mycotoxins. The conidiophore terminates in a ball-like structure called a vesicle. Its conidiospores, which typically appear brown to black (see Fig. 6A), are produced in chains on phialides coming off of the vesicle. (See Fig. 6B and Fig. 6C). Fig. 6D shows conidiospores of Aspergillus as seen with a scanning electron microscope.
Fig. 6A: Aspergillus niger Growing on Saboraud Dextrose Agar
Fig. 6B: Conidiospores of Aspergillus niger
Fig. 6C: Conidiospores of Aspergillus niger
Fig. 6D: Scanning Electron Micrograph of Aspergillus showing Conidiospores Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
By Carlos de Paz (https://www.flickr.com/photos/cdepaz/2421827982/) [CC BY-SA 2.0 (https://creativecommons.org/licenses/by-sa/2.0)], via Wikimedia Commons By Mogana Das Murtey and Patchamuthu Ramasamy ([1]) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
Although generally harmless in most healthy individuals, Aspergillus species do cause allergic bronchopulmonary aspergillosis (ABPA), chronic necrotizing Aspergillus pneumonia (or chronic necrotizing pulmonary aspergillosis [CNPA]), aspergilloma (a mycetoma or fungus ball in a body cavity such as the lung), and invasive aspergillosis. In highly immunosuppressed individuals, however, Aspergillus may disseminate beyond the lung via the blood.
Rhizopus is an example of a mold that produces sporangiospores. Although usually non-pathogenic, it sometimes causes opportunistic wound and respiratory infections in the compromised host. At the end of its sporangiophore is dome-shaped end called a columella that extends into a sac-like structure called a sporangium. Its sporangiospores, typically brown or black (see Fig. 7A), are produced within the sporangium (see Fig. 7B). Anchoring structures called rhizoids are also produced on the vegetative hyphae.
Mucormycoses are infections caused by fungi belonging to the order of Mucorales. Rhizopus species are the most common causative organisms. The most common infection is a severe infection of the facial sinuses, which may extend into the brain. Other mycoses include pulmonary, cutaneous, and gastrointestinal.
Rhizopus can also reproduce sexually. During sexual reproduction (see Fig. 7C), hyphal tips of (+) and (-) mating type join and their nuclei fuse to form a sexual spore called a zygospore (see Fig. 7D). This gives rise to a new sporangium producing sporangiospores having DNA that is a recombination of the two parent strain's DNA.
Fig. 7A: Rhizopus Growing on SDA
Fig. 7B: Sporangiospores of Rhizopus
Fig. 7C: Sexual reproduction of Rhizopus
Fig. 7D: Zygospore of Rhizopus
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Molds are commonly cultured on fungal-selective or enriched media such as Saboraud Dextrose agar (SDA), Corn Meal agar, and Potato Dextrose agar.
B. DERMATOPHYTES
The dermatophytes are a group of molds that cause superficial mycoses of the hair, skin, and nails and utilize the protein keratin, that is found in hair, skin, and nails, as a nitrogen and energy source. Infections are commonly referred to as ringworm or tinea infections and include:
- tinea capitis (infection of the skin of the scalp, eyebrows, and eyelashes) See Fig. 8A.
- tinea barbae (infection of the bearded areas of the face and neck) See Fig. 8B.
- tinea faciei (infection of the skin of the face) See Fig. 8C.
- tinea corporis (infection of the skin regions other than the scalp, groin, palms, and soles) See Fig. 8D.
- tinea cruris (infection of the groin; jock itch) See Fig. 8E.
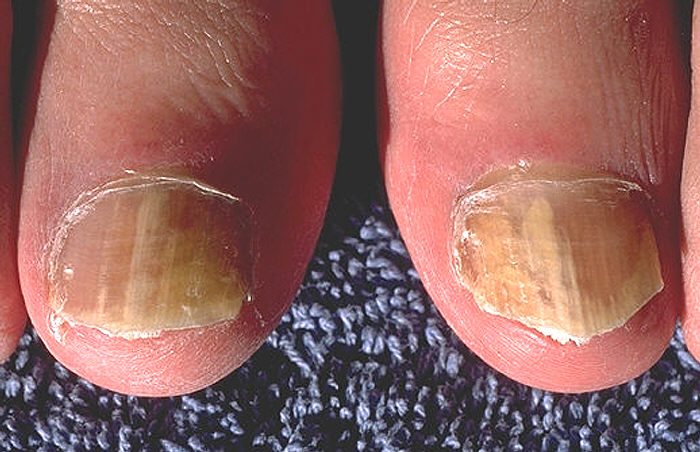
- tinea unguium (onychomycosis; infection of the fingernails and toenails) See Fig. 8F.
- tinea pedis (athlete's foot; infection of the soles of the feet and between the toes) See Fig. 8G..
Fig. 8A: Tinea Capitis
Fig. 8B: Tinea Barbae
Fig. 8C: Tinea Faciei
Fig. 8D: Tinea Corporis
By Content Providers: CDC/Dr. Lucille K. Georg [Public domain].
Courtesy of the Centers for Disease Control and Prevention.By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
Fig. 8E: Tinea Cruris
Fig. 8F: Tinea Unguium
Fig. 8G: Tinea Pedis
By Robertgascoin (https://commons.wikimedia.org/wiki/File:Ji2.jpg) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons By Content Providers: CDC/Dr. Edwin P. Ewing, Jr. [Public domain].
Courtesy of the Centers for Disease Control and Prevention.By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
The three common dermatophytes are Microsporum, Trichophyton, and Epidermophyton. These organisms grow well at 25°C. They may produce large leaf or club-shaped asexual spores called macroconidia (see Fig. 9A) as well as small spherical asexual spores called microconidia, both from vegetative hyphae (see Fig. 9B).
Microsporum (see Fig. 9C) commonly infects the skin and hair, Epidermophyton, the skin and nails, and Trichophyton, the hair, skin, and nails. Dermatophytic infections are acquired by contact with fungal spores from infected humans, animals, or objects. On the skin, the dermatophytes typically cause reddening, itching, edema, and necrosis of tissue. This is a result of fungal growth and a hypersensitivity of the host to the fungus and its products. Frequently there is secondary bacterial or Candida invasion of the traumatized tissue.
Fig. 9A: Macroconidia and Microconidia of Dermatophytes
Fig. 9B: Macroconidia of the Dermatophyte Microsporum
Fig. 9C: The Dermatophyte Microsposum Growing on Saboraud Dextrose Agar
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
To diagnose dermatophytic infections, tissue scrapings can be digested with 10% potassium hydroxide (which causes lysis of the human cells but not the fungus) and examined microscopically for the presence of fungal hyphae and spores. To establish the specific cause of the infection, fungi from the affected tissue can be cultured on Dermatophyte Test Medium (DTM) and Saboraud Dextrose agar (SDA).
Dermatophyte Test Medium (DTM) has phenol red as a pH indicator with the medium yellow (acid) prior to inoculation. As the dermatophytes utilize the keratin in the medium, they produce alkaline end products that raise the pH, thus turning the phenol red in the medium from yellow or acid to red or alkaline. (See Fig. 10A and 10B.).
The types of macroconidia and microconidia (see Fig. 9B above) can be observed by growing the mold on SDA and observing under a microscope. In addition, many dermatophyte species produce yellow to red-pigmented colonies on SDA and the most common species of Microsporum fluoresce under ultraviolet light.
Fig. 10A: An Uninoculated Flask of Dermatophyte Test Medium (DTM)
Fig. 10B: A Dermatophyte Growing on Dermatophyte Test Medium (DTM)
An uninoculated flask of DTM with its acidic pH (yellow).
A dermatophyte growing on DTM. Note alkaline end products.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
C. DIMORPHIC FUNGI
Dimorphic fungi may exhibit two different growth forms. Outside the body they grow as a mold, producing hyphae and asexual reproductive spores, but inside the body they grow as a yeast-like form. Dimorphic fungi may cause systemic mycoses which usually begin by inhaling spores from the mold form. After germination in the lungs, the fungus grows as a yeast. Factors such as body temperature, osmotic stress, oxidative stress, and certain human hormones activate a dimorphism-regulating histidine kinase enzyme in dimorphic molds, causing them to switch from their avirulent mold form to their more virulent yeast form.
The infection usually remains localized in the lungs and characteristic lesions called granuloma (see Fig. 11) may be formed to wall-off and localize the organism. In rare cases, usually in an immunosuppressed host, the organism may disseminate to other areas of the body and be life threatening. Examples of dimorphic fungi include Coccidioides immitis, Histoplasma capsulatum, and Blastomyces dermatitidis.
Fig. 11: Chest X-ray of a Person with Histoplasmosis
Note granuloma, the small white nodules. By Content Providers: CDC/Dr. Lucille K. Georg [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
1. Coccidioides immitis
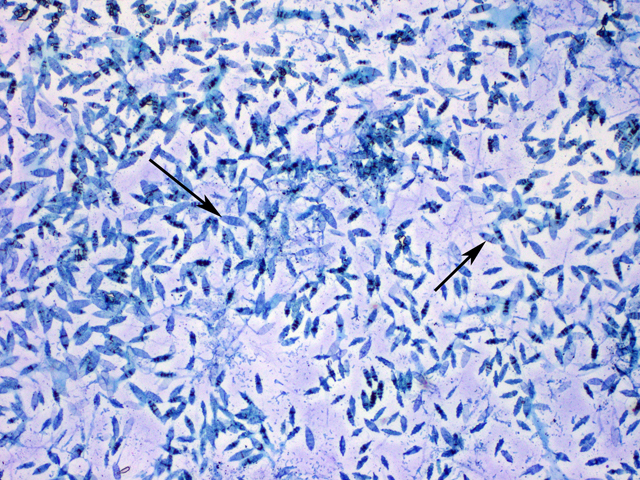
Coccidioides immitis (see Fig. 12A) and Coccidioides posadasii are dimorphic fungi that causes coccidioidomycosis, a disease endemic to areas of the southwestern United States where there is a semiarid climate, an alkaline soil, and hot summers. An estimated 150,000 infections occur annually in the United States, but two thirds of these cases are sub-clinical. The mold form of the fungus grows in arid soil and produces thick-walled, barrel-shaped asexual spores called arthroconidia by a fragmentation of its vegetative hyphae (see Fig. 12B).
After inhalation, the arthroconidia sheds its outer coating, swells, and becomes an endosporulating spherule in the terminal bronchioles of the lungs (see Fig. 12C and Fig. 12D). The spherule contains hundreds to thousands of yeast-like endospores. The spherule subsequently ruptures and releases endospores that develop into new spherules.
Fig. 12A: Life Cycle of the Dimorphic Fungus Coccidioides immitis
By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
Fig. 12B: Arthroconidia of Coccidioides immitis
Fig. 12C: Coccidioides immitis in the Lung: A Spherule Containing Endospores
Fig. 12D: Coccidioides immitis in the Lung: Spherules Containing Endospores
By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.By Content Providers: CDC/Dr. Edwin P. Ewing, Jr. [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
Coccidioidomycosis can be diagnosed by culture, by a coccidioidin skin test, and by indirect serologic tests (discussed in Lab 16).
2. Histoplasma capsulatum
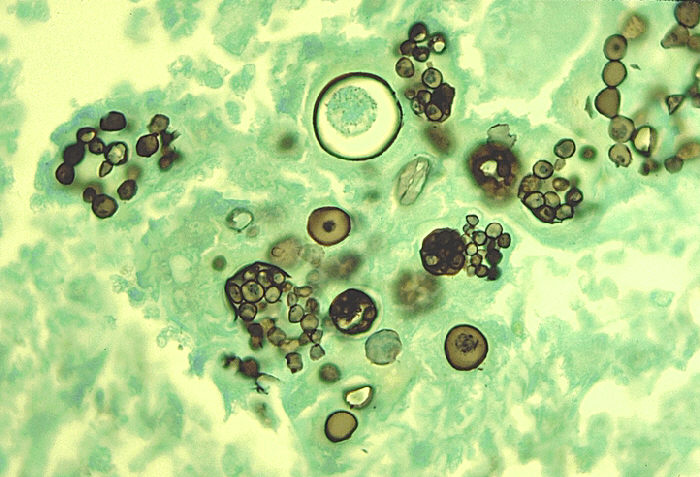
Histoplasma capsulatum (see Fig. 13A) is a dimorphic fungus that causes histoplasmosis, a disease that is endemic to the Ohio, Missouri, and Mississippi River valleys in the United States. Approximately 250,000 people are thought to be infected annually in the US, but clinical symptoms of histoplasmosis occur in less than 5% of the population. Most individuals with histoplasmosis are asymptomatic. Those who develop clinical symptoms are typically either immunocompromised or are exposed to a large quantity of fungal spores.
The mold form of the fungus often grows in acidic, damp soil with high organic content, especially near areas inhabited by bats and birds and produces large tuberculate macroconidia and small microconidia (see Fig. 13B). Although birds cannot be infected by the fungus and do not transmit the disease, bird excretions contaminate the soil and enrich it for mycelial growth. Bats, however, can become infected and transmit histoplasmosis through their droppings. After inhalation of the fungal spores and their germination in the lungs, the fungus grows as a budding, encapsulated yeast (see Fig. 13C).
Fig. 13A: Life Cycle of the Dimorphic Fungus Histoplasma capsulatum
By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
Fig. 13B: Mold Phase of Histoplasma capsulatum Showing Tuberculate Macroconidia and Microconidia
Fig. 13C: Yeast Phase of Histoplasma capsulatum in the Lungs
By Content Providers: CDC/Dr. Libero Ajello [Public domain].
Courtesy of the Centers for Disease Control and Prevention.By Content Providers: CDC/Dr. Libero Ajello [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
Histoplasmosis can be diagnosed by culture, by a histoplasmin skin test, and by indirect serologic tests (discussed in Lab 16).
3. Blastomyces dermatitidis
Blastomycosis, caused by Blastomyces dermatitidis (see Fig. 14A), is most found in the Mississippi and Ohio River valley states and Canada provinces bordering the Great Lakes. Infection can range from an asymptomatic, self-healing pulmonary infection to widely disseminated and potentially fatal disease. Pulmonary infection may be asymptomatic in nearly 50% of patients. Blastomyces dermatitidis can also sometimes infect the skin.
Blastomyces dermatitidis produces a mycelium with small conidiospores (see Fig. 14B.) and grows actively in wet soil that has been enriched with animal droppings, decaying vegetable matter, and rotting wood. When spores are inhaled or enter breaks in the skin, they germinate and the fungus grows as a yeast (see Fig. 14C.) having a characteristic thick cell wall. It is diagnosed by culture and by biopsy examination.
Fig. 14A: Life Cycle of the Dimorphic Fungus Blastomyces dermatitidis
By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
; Fig. 14 B: Mold Form of Blastomyces dermatitidis with Hyphae and Conidiospores
Fig. 14 C: Yeast Form of Blastomyces dermatitidis in the Lung
By Content Providers: CDC/Dr. Leanor Haley [Public domain].
Courtesy of the Centers for Disease Control and Prevention.Blastomyces dermatitidis. Gloria Delisle and Lewis Tomalty, authors.
Licensed for use, ASM MicrobeLibrary.
For a description of antifungal agents used to treat fungal infections, see Chemotherapeutic Control of Fungi in my lecture CourseArc lessons.
Medscape articles on infections associated with organisms mentioned in this lab exercise. Registration to access this website is free. |
A. COMMON MOLDS
1. Using your microscope, observe a prepared slide of Penicillium. Focus first using the yellow-striped 10X objective (100X magnification) and then rotate to the 40X objective (400X magnification). Note the type of asexual spores produced and on what they are borne. Focusing instructions when using the 10X objective can be found in Lab 1.
2. Using your microscope and using the yellow-striped 10X objective (100X magnification), observe a prepared slide of Aspergillus. Note the type of asexual spores produced and on what they are borne.
3. Using your microscope and using the yellow-striped 10X objective (100X magnification), observe a prepared slide of Rhizopus. Note the type of asexual spores produced and on what they are borne.
4. Observe the prepared slide showing the zygospore of Rhizopus produced during sexual reproduction.
B. DERMATOPHYTES
1. Observe the dermatophyte Microsporum growing on DTM. Note the red color from production of alkaline end products indicating that it is breaking down the keratin in the agar. This indicates that the organism is a dermatophyte.
2. Microscopically observe macroconidia and microconidia of Microsporum.
C. DIMORPHIC FUNGI
1. Observe the prepared slide of Coccidioides immitis arthroconidia.
2. Observe the pictures of Coccidioides immitis showing the mold form with arthroconidia seen in the soil as well as the endosporulating spherule form seen in the lungs.
3. Observe the pictures of Histoplasma capsulatum showing the mold form with tuberculate macroconidia and microconidia seen in the soil as well as yeast form seen in the lungs.
RESULTS
A. COMMON MOLDS
Make drawings of the molds as they appear microscopically under high magnification and indicate the type of asexual spore they produce. Also note their color and appearance on SDA.
Type of asexual spore:
Spores are borne on: |
Type of asexual spore:
Spores are borne on: |
|
|
Type of asexual spore:
Spores are found in: |
B. DERMATOPHYTES
1. Describe the results of Microsporum growing on Dermatophyte Test Medium (DTM):
- Original
color of DTM =
- Color following
growth of Microsporum =
- Reason for Color Change =
2. Draw the macroconidia and microconidia of Microsporum.
C. DIMORPHIC FUNGI
1. Draw the arthroconidia of Coccidioides immitis.
2. Draw the mold form and endosporulating spherule form of Coccidioides immitis.
Type of spores seen:
3. Draw the mold form and yeast form of Histoplasma capsulatum.
PERFORMANCE OBJECTIVES FOR LAB 10
DISCUSSION
1. Define the following: hypha, mycelium, vegetative mycelium, and aerial mycelium.
2. Describe the principle way molds reproduce asexually.
3. State the main criteria used in identifying molds.
A. COMMON MOLDS
1. Describe conidiospores and sporangiospores and name a mold that produces each of these.
2. Recognize the following genera of molds when observing a prepared slide under high magnification and state the type of asexual spore seen:
a. Penicillium
b. Aspergillus
c. Rhizopus
3. Recognize Rhizopus zygospores.
B. DERMATOPHYTES
1. Define dermatophyte and list three common genera of dermatophytes.
2. Name four dermatophytic infections and state how they are contracted by humans.
3. Describe macroconidia and microconidia.
4. Describe how the following may be used to identify dermatophytes: potassium hydroxide preparations of tissue scrapings, DTM, and SDA.
5. Recognize a mold as a dermatophyte and state how you can tell when given the following:
a. a flask of DTM showing alkaline products
b. a slide under a microscope or picture showing macroconidia and microconidia.
6. Recognize macroconidia and microconidia.
C. DIMORPHIC FUNGI
1. Define dimorphic fungi and state how humans usually contract them.
2. Name three common dimorphic fungal infections found in the United States, state how they are transmitted to humans, and indicate where they are found geographically.
3. Describe the mold form and the yeast-like form of the following:
a. Coccidioides immitis
b. Histoplasma capsulatum
c. Blastomyces dermatitidis
4. Recognize Coccidioides immitis and its arthroconidia when given a prepared slide and a microscope.
SELF-QUIZ